Case Study
Learn how novel therapeutic activity for ageassociated disease is discovered and developed using Fountain’s platform.
screen > In vitro confirmation testing > In vivo age-associated diseases progeria models > In vivo indication-specific model
Screen
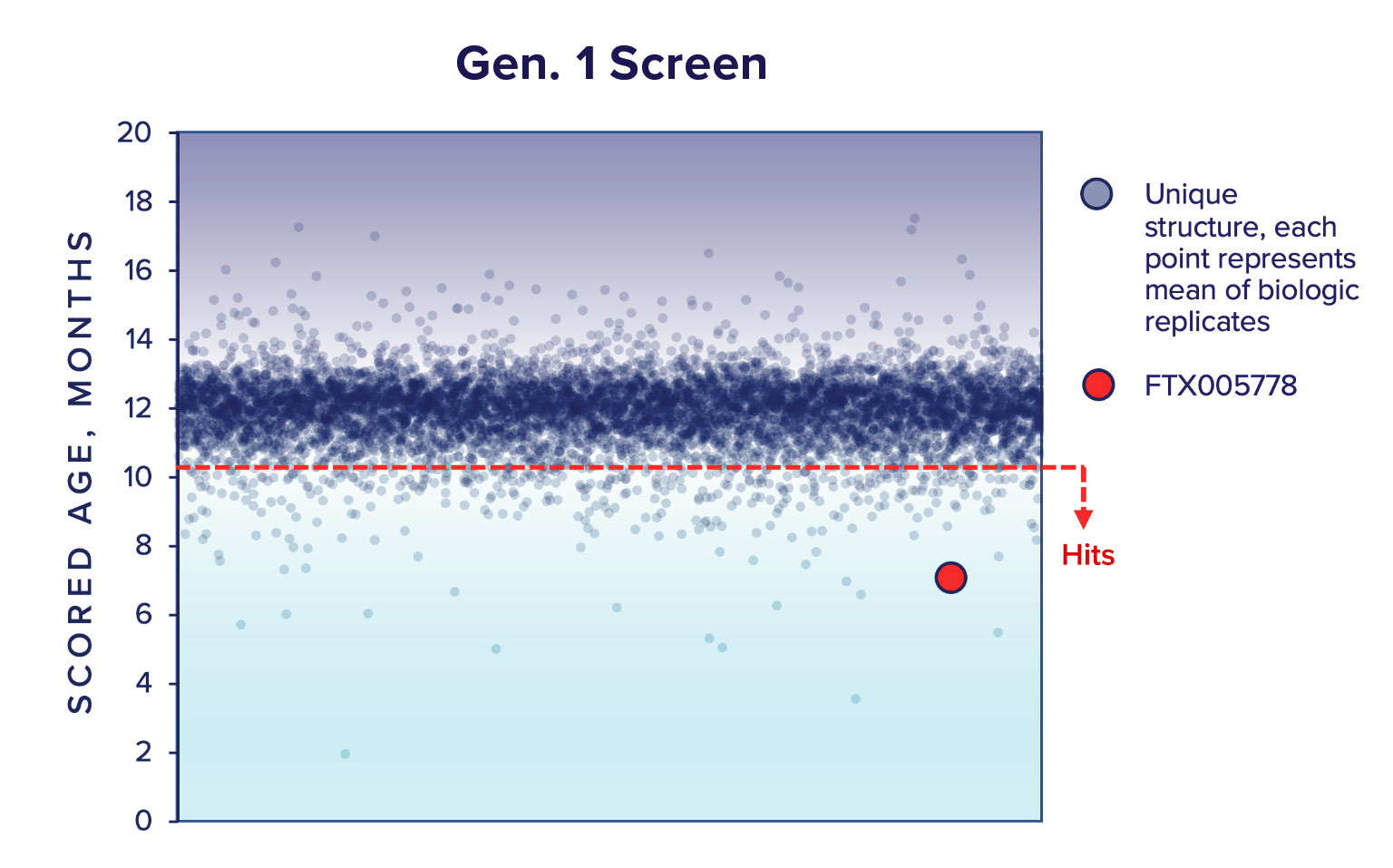
Our Gen.1 compound screen was performed with a library of 8,174 unique drug-like small molecules (Gen.1 library) assembled using criteria on the quality and depth of their target, MoA, in vitro, in vivo, and clinical annotation data. We tested these compounds in an average of 3 biologic replicates (primary cells from different animals, actual range N = 2 – 4). We identified 189 ‘Hits’; compounds that decrease the phenotypic age with a Padj < 0.01.
In vitro confirmation testing
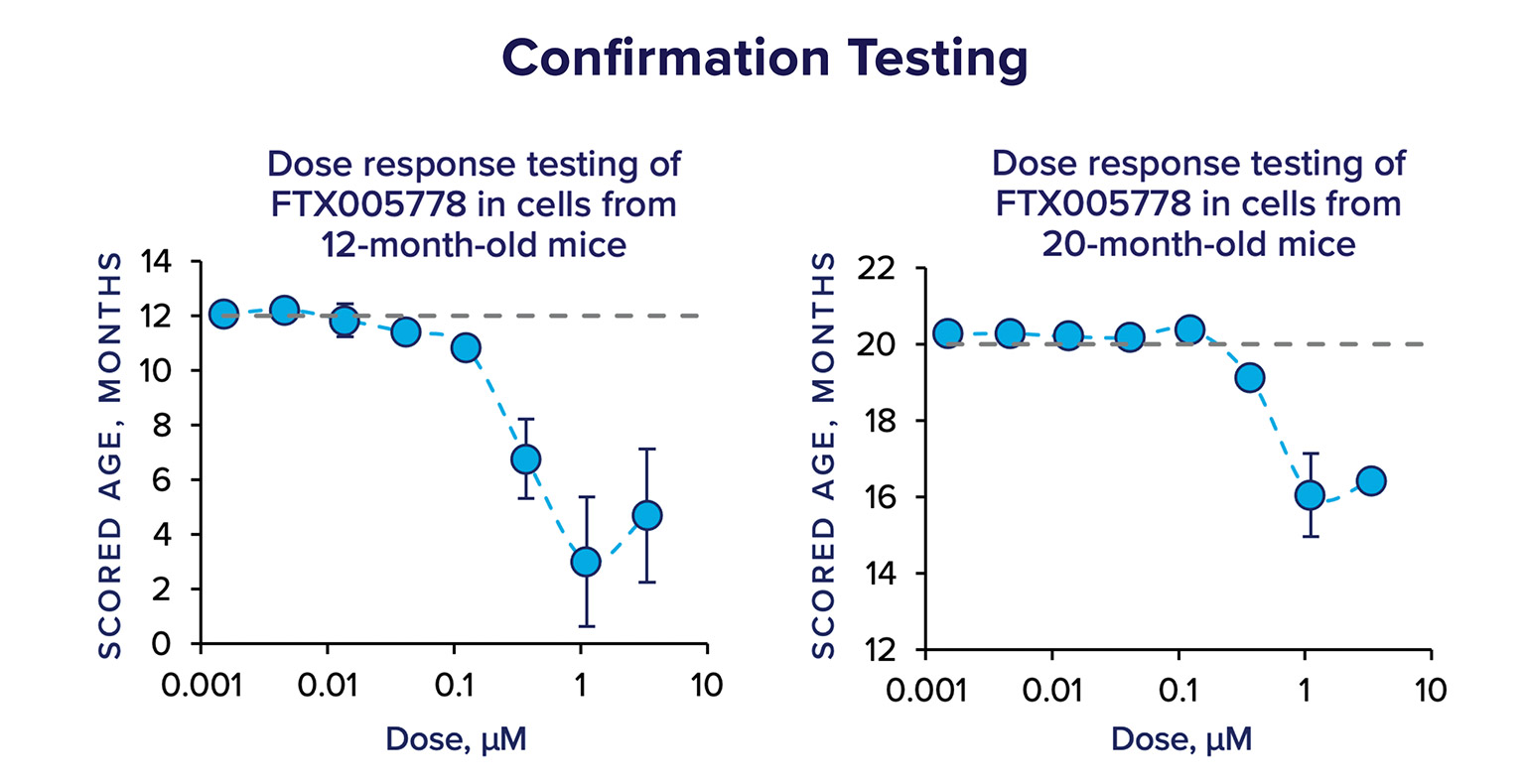
Hits from the primary screen are then subject to confirmation testing; the compounds are re-supplied from additional manufacturers or lots, they are tested at multiple doses, in multiple biologic replicates, and in cells from adult (12-month old) and aged (20-month old) mice. In confirmation testing, FTX005778 had a strong effect on the scored age of cells in a dose-dependent manner.
Using the data from the confirmation testing, we ranked the compounds using a scoring system that incorporated several metrics, including magnitude of the effect, consistency, and area under the curve – and FTX005778 was the highest ranked hit in the Gen.1 screen.
In vivo age-associated diseases progeria models
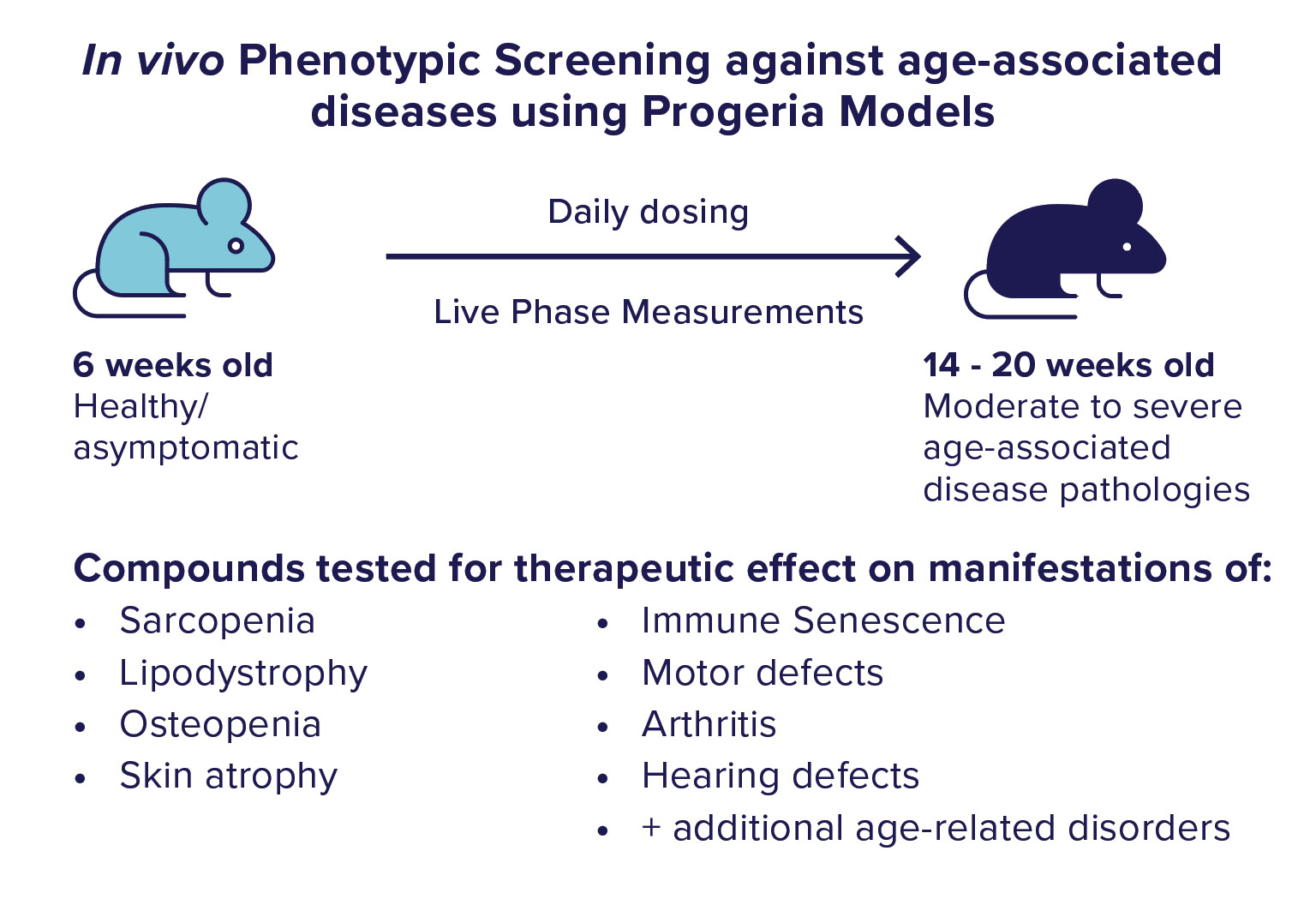
We use the ranking from the confirmation testing to select molecules to move into the in vivo phase of screening. In the in vivo phase of screening. We utilize a mouse model of progeria syndrome to test our “Hit” molecules for therapeutic effects against the development of age-associated diseases. In this mouse model, animals are healthy until about 6 weeks of age when they start to develop many symptoms, pathologies, and conditions of aging. By 15 weeks of age, these animals display moderate to severe pathology. We start dosing the animals in the asymptomatic stage and monitor the progression of disease development using many vital, functional, and physiologic measurements. Additional detailed phenotypic analysis is conducted after the completion of chronic dosing.
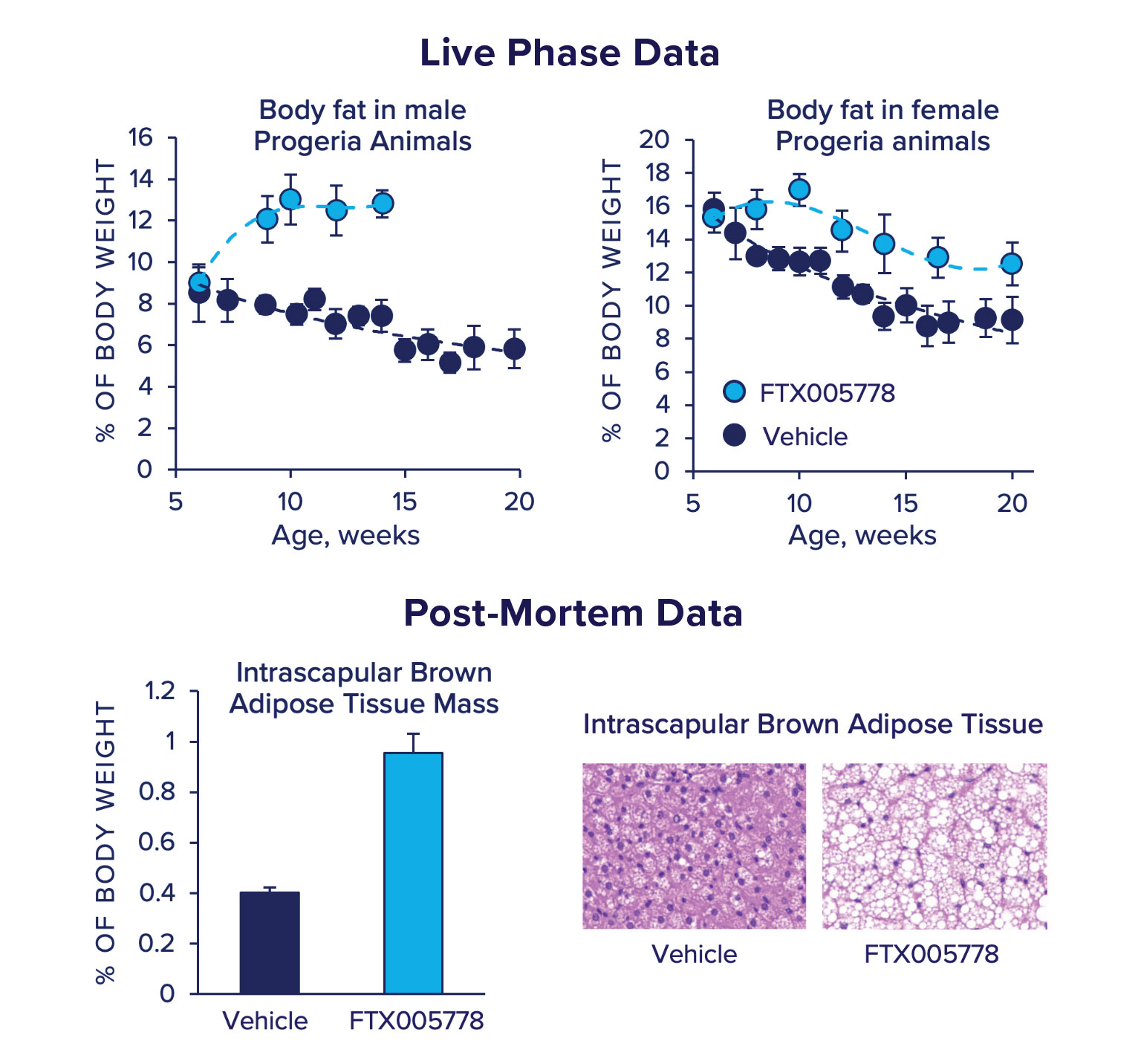
FTX005778 Promotes beneficial lipid storage.
Starting at 6 weeks of age, progeria animals develop startling lipodystrophy and lipoatrophy, which can be monitored in the live phase of dosing by performing TD-NMR-based body composition analyses. We found that animals dosed orally with FTX005778 displayed significant preservation of body fat compared to the decline experienced by animals dosed with vehicle. FTX005778 induced a modest increase in body fat that was stable over the course of dosing in male progeria animals and delayed the decline in body fat in female animals.
Subsequent analyses found that animals dosed with FTX005778 had a profound increase in intrascapular brown adipose tissue mass. Brown adipose tissue is a highly metabolic adipose tissue that is central to metabolic and thermogenic homeostasis. It is well established that brown adipose mass positively correlates with ‘healthy’ metabolic states and declines with age. At 15 weeks of age, the brown adipose tissue in progeria animals dosed with vehicle was atrophied and almost completely devoid of lipid. However, the brown adipose tissue of 15-week old animals dosed with FTX005778 is 2-3 times the mass of vehicle treated and histologically appear normal/healthy: it is comprised of large multilocular adipocytes.
In vivo indication-specific model
We next tested FTX005778 in a model of metabolic disease where diet-induced changes in lipid storage cause disease development and progression. The model used was one in which a high fat, sucrose, and cholesterol diet causes central accumulation of fat, in the form of liver steatosis and visceral adipose accumulation, which then leads to the development of fatty-liver disease, non-alcoholic steatohepatitis, and insulin resistance (Taconic model: NASH B6).
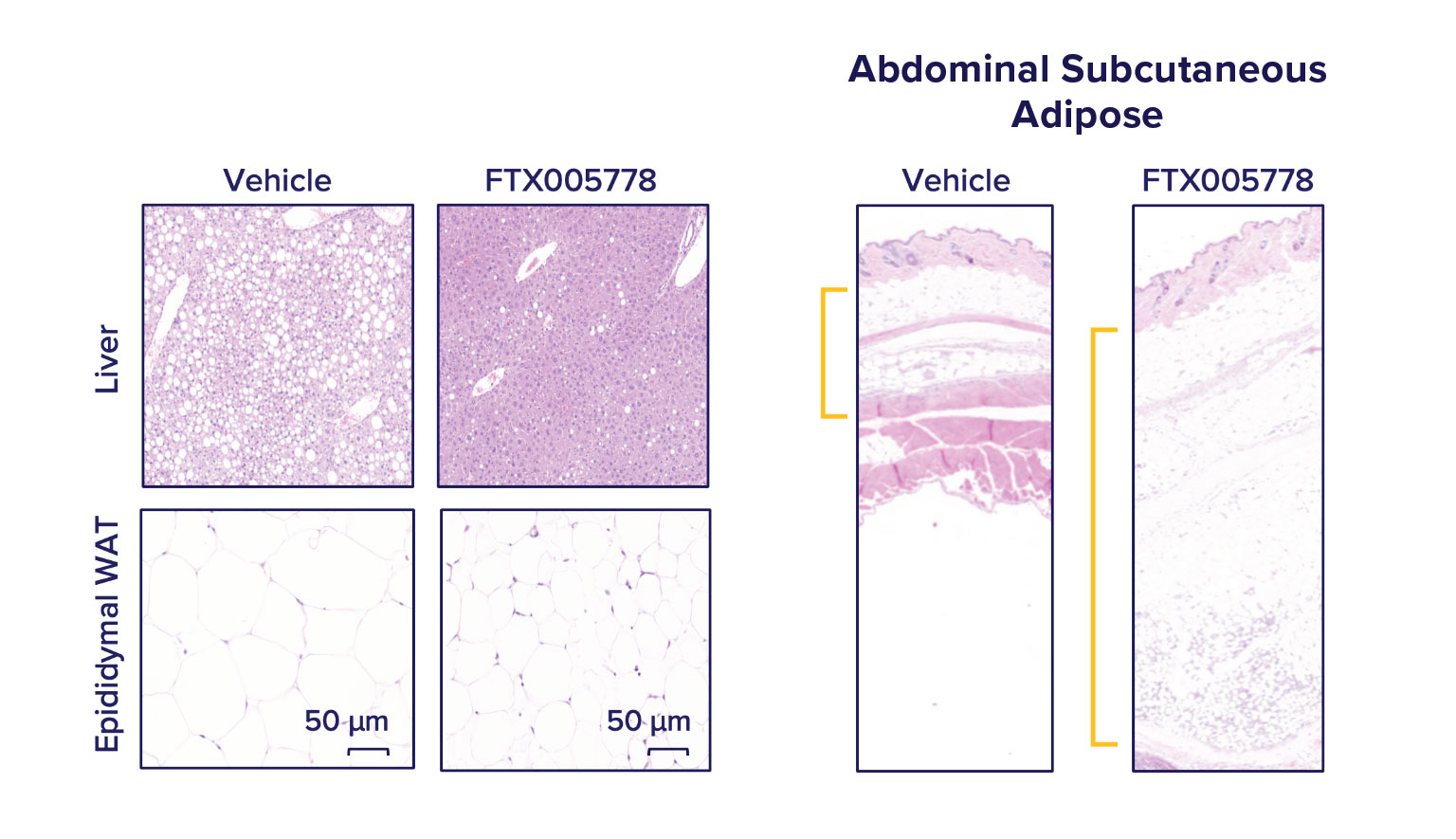
FTX005778 promotes the mobilization of lipid into peripheral depots.
After 4 weeks of treatment, while continuing the high fat, sucrose, cholesterol diet, we found that animals dosed with FTX005778 displayed dramatic reductions in liver steatosis (liver fat) and epididymal (visceral) adipocyte size and tissue mass compared to vehicle-dosed animals. While animals on FTX005778 weighed modestly less than vehicle treated animals, we found there was large expansion in subcutaneous fat.
Storage of lipid in peripheral depots (such as subcutaneous) and brown adipose depots is strongly associated with healthier metabolic states compared to storage in central depots (organ steatosis and visceral adipose). Peripheral adipose, especially subcutaneous, is highly insulin sensitive. It is better able to respond to the body’s energetic needs than insulin-resistant depots (such as visceral adipose and organ steatosis).
FTX005778 is a compound that was developed for applications in solid tumor oncology. However, it failed at efficacy in Phase 3 trials without significant adverse effects. In the literature there have no been previous implications of FTX005778 nor it’s molecular target in whole-body adipose biology. Fountain’s platform led to the discovery of a potential therapeutic application of FTX005778 for age-associated lipodystrophy and diet-induced central obesity. For strategic reasons, Fountain has decided not to pursue this molecule to focus efforts on other higher priority programs.
In summary, Fountain’s proprietary platform is built to use cell phenotype to discover and develop therapies towards diseases of aging. Specifically, Fountain’s platform enables:
- Discovery of novel targets, pathways, and drugs for diseases of aging
- Identification of novel therapeutic activity of developed/known targets and drugs
- A customizable approach for unbiased drug and target discovery in disease-specific and cell-type specific systems.
